Carbon Monoxide Ionic Or Covalent
nine.eight: Coordinate Covalent Bond
- Folio ID
- 53748
Is sharing a practiced thing?
Remember when you were younger, and were told to share your favorite toy with your blood brother, sister, or friend? You probably didn't want to share, but did anyway. It likely turned out that you had more fun playing with the toy together than if you had kept information technology to yourself. Atoms often share electrons with other atoms that have zippo to contribute to the situation; the terminate result is a new structure.
Coordinate Covalent Bonds
Each of the covalent bonds that we accept looked at so far has involved each of the atoms that are bonding contributing i of the electrons to the shared pair. At that place is an alternate type of covalent bond in which 1 of the atoms provides both of the electrons in a shared pair. Carbon monoxide, \(\ce{CO}\), is a toxic gas that is released as a byproduct during the called-for of fossil fuels. The bonding betwixt the \(\ce{C}\) atom and the \(\ce{O}\) cantlet can exist thought of in the post-obit procession:
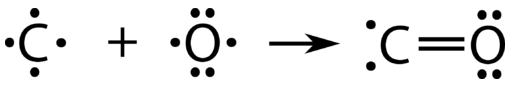
At this point, a double bail has formed between the 2 atoms, with each atom providing one of the electrons to each bond. The oxygen atom now has a stable octet of electrons, but the carbon atom only has vi electrons and is unstable. This situation is resolved if the oxygen atom contributes one of its lone pairs in order to brand a third bail with the carbon atom.
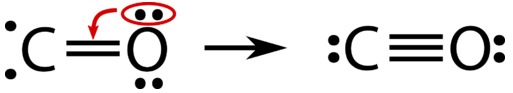
The carbon monoxide molecule is correctly represented past a triple covalent bond betwixt the carbon and oxygen atoms. Ane of the bonds is a coordinate covalent bail, a covalent bail in which i of the atoms contributes both of the electrons in the shared pair.
Once formed, a coordinate covalent bond is the same as any other covalent bond. It is not equally if the two conventional bonds in the \(\ce{CO}\) molecule are stronger or dissimilar in whatever other mode than the coordinate covalent bond.
Summary
- Coordinate covalent bonds can form when 1 atom provides a lone pair of electrons to the bail.
- Coordinate covalent bonds are as stiff as other covalent bonds.
Review
- Where does the 3rd covalent bail in the CO molecule come from?
- Why is the wrong structure for CO above wrong?
- Are coordinate covalent bonds stronger or weaker than regular covalent bonds?
Carbon Monoxide Ionic Or Covalent,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/09%3A_Covalent_Bonding/9.08%3A_Coordinate_Covalent_Bond#:~:text=The%20carbon%20monoxide%20molecule%20is,electrons%20in%20the%20shared%20pair.
Posted by: ellingtonmorold90.blogspot.com


0 Response to "Carbon Monoxide Ionic Or Covalent"
Post a Comment