No3 1 Lewis Dot Structure
Nitrogen Dioxide (NOtwo) is a covalent compound that is composed of a primal nitrogen cantlet single bonded to an oxygen cantlet and a double bail with another oxygen atom. At room temperatures, nitrogen dioxide is a ruby-brown gas that has a density of 1.eight g/dmiii. Information technology is slightly toxic to humans, on business relationship of its tendency to react in the human trunk and produce reactive species of nitrogen and oxygen, which tin can damage internal structures.
"A small bubble of air remained unabsorbed… if there is any function of the phlogisticated air [nitrogen] of our atmosphere which differs from the rest, and cannot exist reduced to nitrous acid, we may safely conclude it is not more than 1/120 part of the whole." — Henry Cavendish
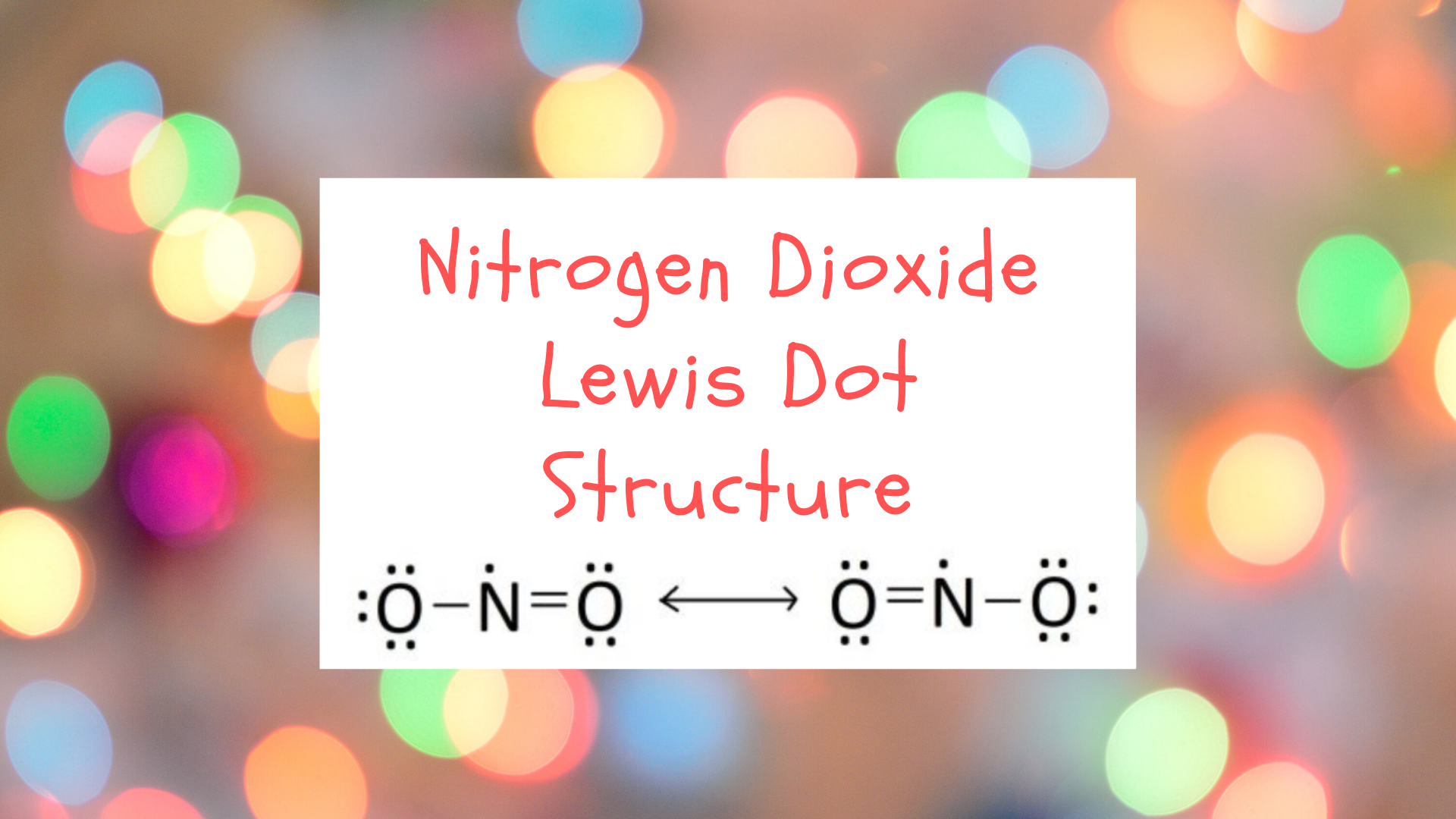
Nitrogen dioxide does not take a single Lewis structure on business relationship of its relatively strange electron configuration. The location of the double bond changes over time, meaning that at any signal, either of the oxygen atoms could have a double bond with the nitrogen atom. As such, nitrogen dioxide is represented by the resonance Lewis structure:

Nitrogen dioxide requires a resonance Lewis structure because its electron configuration constantly oscillates betwixt the ii forms. The "true" electron configuration of nitrogen dioxide is considered to be some boilerplate of the two resonance structures given above. The Lewis structure of nitrogen dioxide is also interesting because in that location is a unmarried unpaired valence electron on the central nitrogen cantlet. Compounds with unpaired electrons are sometimes referred to as "free radicals." This unpaired electron explains nitrogen dioxide's reactive behavior every bit it has a stiff desire to fill this open electron spot.
Let's have a step back and get over the rules for cartoon a Lewis structure. Nosotros will go stride-by-step to see how we can construct a Lewis construction for nigh main-grouping compounds, nitrogen dioxide included.
Lewis Structures: The Nuts
In a nutshell, a Lewis structure is a pictorial representation of the atomic construction and electron configuration of an atom or a compound. Single atoms are represented by their unique chemic symbol, electrons are represented equally single dots, and shared pairs of electrons are represented past a single dash (−) for a single pair, a double bar (=) for a double pair, and a triple bar (≡) for a triple pair.
"Every chemical substance, whether natural or artificial, falls into one of ii major categories, according to the spatial feature of its form. The distinction is betwixt those substances that have a airplane of symmetry and those that practise non. The former vest to the mineral, the latter to the living world." — Louis Pasteur
The purpose of a Lewis structure is to see how the electrons are arranged in an cantlet or compound. Lewis structures are based on the octet rule—the empirical observation that atoms tend to grade bonds until they accept a complete valence vanquish of 8 electrons. The only exception to the octet rule is hydrogen, which volition only form bonds until it has 2 valence electrons.
Valence electrons are represented as pairs of dots, where each dot represents a unmarried electron. Atoms grade covalent bonds by sharing their valence electrons with other atoms. For example, a single chlorine atom has 7 valence electrons; 3 pairs and one free electron. 2 chlorine atoms volition share their unpaired electron and then that each atom has a full octet of electrons, forming a chlorine molecule (Cltwo). In full general, this is how covalent bonds work. Atoms volition share valence electrons until each cantlet has a full octet. If all the valence electrons are paired, but an cantlet still does non have a full octet, electron pairs will move to form double and triple bonds. The full amount of electrons in a Lewis structure is equal to the sum of the number of valence electrons of the individual atoms.
Lewis structures tell you about the atomic organization and electron distribution of an atom or compound. While Lewis structures alone exercise not requite explicit information well-nigh the 3-D geometry of a molecule, the rules for writing Lewis structures can be combined with rules governing molecular geometry to predict the shape a compound volition have.
Rules for Making Lewis Structures
Let's become through the rules for making Lewis structure, using nitrogen dioxide as our test case.
Step i. Determine the full amount of valence electrons.
The first step is to effigy out how many electrons you diagram should accept. The total number of electrons in a Lewis structure should be equal to the sum of the valence electrons of each individual atom. The number of valence electrons of an element can exist determined by looking at their group number on the periodic table. In full general, groups i and 2 elements have one and two valence electrons, respectively. Grouping 13-18 elements accept 3, 4, 5, six, 7, and 8 valence electrons respectively. Group 3-12 elements are the transition metals which can have dissimilar amounts of valence electrons
In our case, nitrogen dioxide is equanimous of 1 nitrogen atom and 2 oxygen atoms. Nitrogen is a group 15 element so has 5 valence electrons, while group 16 oxygen has 6 valence electrons. There are two oxygen atoms, then the total corporeality of valence electrons in our diagram is:
5(1) + 6(ii) =17 electrons
Our diagram should have 17 electrons in full.
Step ii: Sketch the atomic construction of the compound
Now that nosotros accept the number of valence electrons, nosotros can brainstorm constructing the diagram. If the compound is diatomic (ii atoms) the structure is easy; the atoms tin can be placed next. In the instance of compounds with three or more than atoms, there is normally a fundamental atom that shares multiple bonds with terminal atoms. In general, for triatomic or grater compounds, the primal cantlet is the least electronegative chemical element.
In our case, nosotros have a triatomic chemical compound so our structure almost likely volition have a fundamental atom bonded to multiple terminal atoms. Nitrogen is less electronegative than oxygen (iii.04<3.44), so nitrogen is our central. Placing the symbols give us:
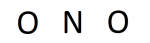
Credit: Author
Stride iii: Place pairs of electrons so that every atom has at least one unmarried bail
Side by side up nosotros can get-go placing electrons in our model. First, we become through and place a single bond between each cantlet. Every single bond counts for 2 electrons, and so we decrease those electrons from out total amount to get how many we have left to place.
In our case, we place two single bonds, 1 between each atom, which looks like:

Since we placed ii pairs, nosotros placed iv electrons in total. We now have 17-4 =13 more electrons to place.
"Modern chemistry, with its far-reaching generalizations and hypotheses, is a fine example of how far the human mind can get in exploring the unknown beyond the limits of senses." — Horace G. Deming
Step iv: Place electron pairs, starting with the terminal atoms, until each has a full octet.
Next, we place the remaining electrons. Commencement with the terminal atoms, fill in dots until each atom has a total of 8 valence electrons. If you have whatever leftover atoms, place them on the key cantlet in pairs or every bit lone electrons.
Offset with the terminal oxygens starting time, we place 6 electrons around each so that they have a total octet. Whatsoever leftover electrons we place on the nitrogen cantlet. Adding vi electrons to each oxygen atoms is 12 total, so we place the remaining single electron on the nitrogen atom:

Subsequently placing these 13 electrons, we now have thirteen-13 =0 electrons left to place. But, we are not done still because our central cantlet however does not have a full octet, nitrogen currently only has 5 electrons; 2 pairs and a single unpaired electron.
Stride 5: Move electron pairs to course double and triple bonds until each atom has an octet, or is as close as it can get to an octet.
If all the electrons have been placed and some atoms still do non have a full octet, then compounds volition form double and triple bonds to brand certain every atom gets equally close to viii electrons as possible. Simply move electron pairs from terminal atoms to make double and triple bonds.
In our case, we take all of our electrons placed purchase nitrogen only has five valence electrons. Moving an unbonded electron pair from one of the oxygen atoms creates a double bail with nitrogen, giving it 7 electrons. Moving any more than electron pair would give nitrogen more than 8 electrons, so we have gone as far as nosotros can and our Lewis construction should look like this:
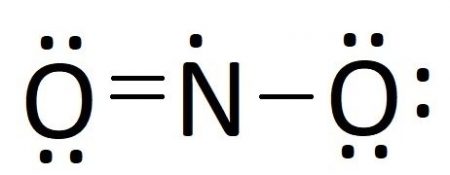
Resonance Structures
In the last footstep of drawing our Lewis diagram, nosotros needed to cull an electron pair to motion to make a double bond. We picked the left oxygen atom, but couldn't we have picked the right oxygen atom to get something like this?:

The answer is yes, this is too a valid Lewis structure for nitrogen dioxide. However, this structure is plain unlike for the previous; the double bail is on the right instead of the left. If both Lewis structures are legitimate, and then which one is the "actual" Lewis structure of nitrogen dioxide? The answer is:both.
In cases where there is more than one legitimate Lewis construction for a chemical compound, the entire Lewis construction is represented as an average of the multiple structures. These structures are known every bitresonance structures and they are used for compounds whose electron configuration cannot be completely represented by a unmarried unique Lewis diagram. A resonance structure for our two diagrams for nitrogen dioxide looks similar:

The "actual" structure of nitrogen dioxide is interpreted as some combination of the two diagrams. Resonance structures are possible because, for some compounds, electron pairs aredelocalized and oscillate betwixt i configuration and another. Resonance structures are required because some molecules' atomic configurations cannot be accurately captured with a single Lewis structure.
Limitations Of Lewis Structures
Following the rules of Lewis structure should allow you to construct a Lewis structure for most compounds fabricated from main grouping elements in the s- and p-blocks of the periodic table. Some families of elements do not always obey the rules for making Lewis structures. Transition metals, for instance, oftentimes do not follow the octet rule and tin bail and become up to 12 valence electrons. The further down the periodic table 1 goes, the less and less the basic rules for Lewis structures use due to the extremely heavy nuclei and strong electromagnetic properties of large elements.
No3 1 Lewis Dot Structure,
Source: https://sciencetrends.com/no2-nitrogen-dioxide-lewis-dot-structure/
Posted by: ellingtonmorold90.blogspot.com


0 Response to "No3 1 Lewis Dot Structure"
Post a Comment